Learning Objectives
- What oomycetes are, and how they damage plants
- Symptoms of oomycete diseases
- Potential exotic disease threats to Alaska
- How to report or submit samples for diagnosis
- Vocabulary associated with oomycete diseases
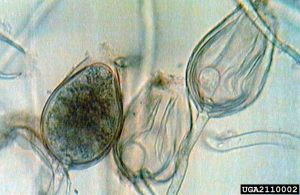
Oomycetes, also known as “water molds”, were once thought to be fungi. They are so different in their composition and evolutionary history, they are now considered in the Kingdom Chromista. They produce tough survival structures known as oospores, and many species also produce sporangia, containing swimming zoospores.
There are several hundred species of oomycetes, causing plant diseases such as damping off, blights, root and stem rots, and downy mildews. One of the most infamous plant disease epidemics, the Irish Potato Famine, was caused by Phytophthora infestans (cause of late blight). “Phytophthora” means “plant destroyer”; this genus contains over 60 species, many are important pathogens around the world.
Exotic Oomycete Pathogens
Sudden oak death and Ramorum leaf blight (caused by Phytophthora ramorum)
Phytophthora ramorum causes sudden oak death, ramorum leaf blight, and ramorum dieback. Its primary threat is to forest ecosystems, along with regulatory costs and quarantines. Over 1 million trees have been lost in coastal California. P. ramorum was first observed in Germany and the Netherlands in 1993, and California in the mid 1990’s. It has been found in forests of California and Oregon; it has also been found in nurseries in California, Oregon, Washington, and British Columbia. A 2004 distribution of infected nursery stock resulted in detection and eradication in 21 states. In 2006, P. ramorum was detected in 62 nurseries in 11 states. As of 2011, 464 nurseries in 27 states have found the pathogen. There have been at least four global migrations, three in North America, and one in Europe. The economic impact of P. ramorum has been in the tens of millions of dollars in the United States. Losses have occurred in destroyed plant material, export markets, tourism effects, prevention costs, management and eradication costs, loss of wildlife habitat, increased fire risk, hydrologic changes, changes in forest structure, and lost aesthetic value.
P. ramorum was probably recently introduced to North America, due to its limited geographical distribution, relative to the wide distribution of host species. P. ramorum is spread by wind-blown rain, irrigation water, infested soil or potting mix, and movement by humans. Moist, cool, windy conditions help spread spores. The temperature range is 36-80°F, with optimum growth at 68°F. Temperature and humidity determine disease levels.
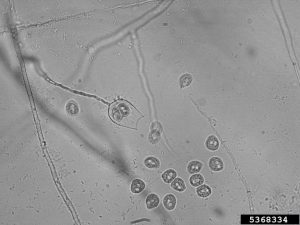
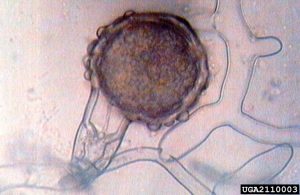
P. ramorum produces multiple reproductive structures. Sporangia are asexual structures that can germinate directly to infect hosts; they can also produce swimming zoospores, which swim through water films on leaves or in soil before infection. Infected tissue can give rise to new sporangia (and more cycles of infection), or tough chlamydospores to survive difficult environmental conditions (drought, winter, etc.) up to 650 days. Sexual reproduction can occur when different mating types are present in an area. This produces durable oospores, capable of surviving difficult conditions, as well as generating new genetic variability.
Hosts and Symptoms
P. ramorum has a large host range, with new hosts regularly identified. Newly emerging pathogens may expand their host range by migration to new areas with previously unknown susceptible hosts, introduction of new hosts to an infested area, or hybridization with related species. The host range of P. ramorum includes over 100 species, in over 40 genera. This includes many native and domesticated plants commonly found in Alaska, as well as in the nursery and greenhouse trade. There are ranges of susceptibility within species and sometimes cultivar.
Infection with P. ramorum can cause different symptoms in different hosts. In oaks and tanoaks, bleeding cankers can be formed, with red to black sap oozing on the bark surface; the host may be killed. Most of the hosts expected in Alaska (ex. rhododendron, lilac, and cranberry; see Table 1 below) display foliar lesions, leaf blight, twig dieback, or stem cankers. Foliar hosts are important for the survival and spread of P. ramorum. Some foliar hosts produce large numbers of spores, which are easily spread by rain splash or wind. Root infections are also possible, but may remain symptomless for many months.
In general, leaf symptoms are necrotic leaf lesions with irregular shaped borders. Typically, no fruiting bodies (spore-producing structures) are visible. Leaf infection can develop down petioles into twigs, but leaves often drop before lesions reach petioles. Since P. ramorum requires liquid water for zoospores to swim, lesions are often found where water accumulates: leaf tips, depressions, overlapping or cupped leaves, inner canopies, and leaves near or touching soil.
| Common Name | Genus species | Symptoms |
| Japanese larch | Larix kaempferi | Leaf necrosis; dieback; canker with resin |
| Rhododendron | Rhododendron spp. (all species) | Leaf necrosis esp. leaf edges near petiole and tip; diffuse margins; premature drop; brown to black cankers on small branches |
| Lilac | Syringa vulgaris | Leaf lesions brown to black esp. leaf edges; death of leaf buds |
| High bush cranberry | Viburnum spp. (all species) | Leaf necrotic lesions at tip, edge, petiole; leaves may drop; stem cankers near base and small branches; distant leaves brown and stay attached |
| Kinnikinnik | Arctostaphylos uva-ursi | Leaf blight and twig dieback; stem and root rots |
| Holly | Ilex (multiple species) | Leaf blight |
| Ninebark | Physocarpus opulifolius | Leaf blight |
| Rose | Rosa (multiple species) | Necrotic leaf lesions, sometimes with chlorotic halo, spreading down petiole |
| Salmonberry | Rubus spectabilis | Leaves with dark marginal necrosis |
| Lingonberry, Low bush cranberry | Vaccinium vitis-idaea | Leaf lesions dark, margins diffuse |
| Veronica | Veronica spicata | Leaf necrosis |
| Fireweed | Chamerion angustifolium | Leaf lesions irregular shaped, may be vein-delimited |
| Northern willowherb | Epilobium ciliatum | Leaf lesions |
| Camellia | Camellia spp. (all species) | Water-soaked leaf lesions on tip or edge, diffuse margins or thick black zone lines, premature leaf drop |
| Andromeda | Pieris spp. (all species) | Similar to Rhododendron; branch tip dieback, premature leaf drop |
Table 1. Native and Domesticated Phytophthora ramorum Hosts in Alaska
Symptoms may resemble fungal infections, freeze damage, drought, fertilizer burn, chemical injury, sunburn, or root damage. Abiotic injuries often cause lesions with distinct margins, and may affect the entire plant. P. ramorum usually causes lesions with diffuse margins, and damages a few leaves or part of a plant. [see https://ppo.puyallup.wsu.edu/sod/education/mginfo/pr-symptoms/ ] Symptoms are not sufficient for diagnosis and require testing for determination.
A range of diagnostic tests are available, some are generic for any Phytophthora (i.e. species can’t be determined), these include onsite “dipstick” (lateral flow) devices, and Enzyme-Linked Immunosorbent Assay (ELISA). Species identity can be determined by growing a culture on special media, or conducting sensitive molecular testing, such as Polymerase Chain Reaction (PCR), or one of its variants.
P. ramorum is introduced to new areas in infected plant materials and infested soil. Windblown spores and infested water are responsible for more localized spread. Exclusion of P. ramorum requires large geographic barriers, due to windblown spores. Quarantines restrict movement of infected host material. A U.S. Federal Order in 2012 (DA-2012-14), addresses restrictions on importation of P. ramorum host plants for planting into the United States. This requires phytosanitary certificates or pest exclusion plans from the exporting country. Nursery inspections and sampling aid in containment. Eradication procedures are followed if the pathogen is detected, with trace-back and trace-forward to determine origin and destination.
Good sanitation practices (sterile media, disinfection of tools, treating irrigation water) and nursery/greenhouse Best Management Practices (BMPs) help with all pathogens. Some excellent references for BMPs are in the reference section. Of special note, it is recommended not to use systemic fungicides (mefenoxam, metalaxyl, phosphites or phosphonates) to manage Phytophthora spp. in a nursery or greenhouse. These chemicals slow growth, but do not kill Phytophthora. This masks symptoms and can contribute to spreading these pathogens.
While P. ramorum is found in the U.S., it has a limited distribution at present, and is not found in Alaska. This has cost millions of dollars and much effort to achieve. Introduction of the pathogen to Alaska would be very expensive to manage, and potentially devastating to our nurseries, greenhouses, and natural environment.
For a printable version of information on ramorum leaf blight view and download our factsheet here.
Alder root and collar rot (caused by Phytophthora alni)
Another oomycete of concern is Phytophthora alni, cause of alder root and collar rot. This disease was first discovered in 1993 killing alders in Great Britain, and identified as a new species in 2004. Currently, P. alni is considered a species complex of 3 subspecies: alni, multiformis, and uniformis. P. alni subsp. alni (Paa) is considered more aggressive than P. alni subsp. multiformis (Pam) or P. alni subsp. uniformis (Pau). Paa is frost sensitive, while Pau is cold tolerant. Overall, P. alni is favored by mild winters and warm summers (70- 86°F).
P. alni has caused alder dieback and mortality across Europe. It was probably spread by infected nursery stock and infested irrigation water. Generally, there has been higher incidence (rate of occurrence) in riparian zones (next to rivers and streams), but it has been found away from waterways. Stresses caused by flooding can increase damage.
Alders are important pioneer species, capable of fixing atmospheric nitrogen. They tolerate wet soils, provide shade and cooler water temperatures, provide habitat for aquatic and terrestrial organisms, contribute significant amounts of nitrogen to stream food webs, and stabilize streambanks. Changes in riparian tree species can increase soil erosion, alter currents, and affect fish habitat (including salmonids).
Widespread dieback and mortality of thinleaf alder in Alaska led to surveys in 2007. Phytophthora alni subsp. uniformis (Pau) was discovered near Cooper Landing and Denali National Park, in remote, unmanaged stands. Further surveys found populations near Fairbanks and Tok. However, root and collar rot symptoms were rarely found, suggesting dieback due to other factors. Genetic diversity studies indicate the Alaska Pau isolates are probably indigenous to North America. The risk of introduction of the more aggressive forms still exists.
Hosts and Symptoms
Native and domesticated P. alni hosts include: European or black alder (Alnus glutinosa), gray alder (A. incana), thinleaf alder (A. incana subsp. tenuifolia), green alder (A. viridis), mountain alder (A. viridis subsp. crispa), Sitka alder (A. viridis subsp. sinuata), Italian alder (A. cordata), red alder (A. rubra), and sweet cherry (Prunus avium, experimentally).
P. alni doesn’t appear to produce chlamydospores (tough, asexual spores), but does produce oospores (tough, sexual spores). Sexual recombination allows for greater genetic variability. Swimming zoospores are produced, which can infect fine roots, as well as bark near the root collar.
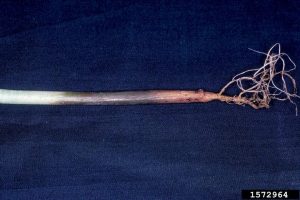
Injuries allow pathogen entry, but P. alni does not require injury to initiate infection. Root collar tissue is killed, resulting in stem girdling. When most of the root collar has been girdled, foliar and crown symptoms start to appear. This may take up to 10 years. Leaves in mid to late summer may be small, yellow and sparse; they may drop prematurely. Crown dieback can occur, with twigs dying in the upper or outer crown.

Tarry spots or rusty exudates may form on the lower portions of the stem. Underlying bark is dead.

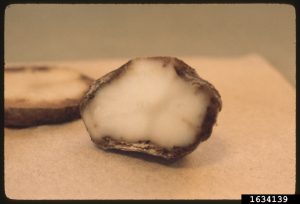
Peeling away the bark may reveal orange-brown necrotic (dead) inner bark in a tongue pattern. Older inactive lesions are dark brown.
In advanced stages, live strips of bark may produce new shoots. Adventitious roots may form above bark lesions on the stem, as the vascular system is damaged. Early or excessive cone production may occur, cones are unusually small. Many symptoms are similar to those caused by other root and collar infecting Phytophthora spp., Pythium spp., and several fungi. As with P. ramorum, identification to genus-level is possible with “dipstick” field kits, identification to species level requires submission to a laboratory.
Spores are spread by movement of plants, rain splash, infested soil, flood and irrigation water. Vehicles and footwear used in high-risk areas (streamside or near diseased alders) should be cleaned and disinfected to reduce movement of infested soil. Outside a living host, spores in soil probably survive less than one year. Recently dead trees (1-3 years) produce little inoculum. Nurseries should use pasteurized or sterilized soil and clean pots. Careful inspection of incoming plant materials helps to exclude diseased material. Periodic scouting allows for early detection and eradication. A 2009 Federal Order (DA-2009-27) prohibits import of Alnus spp. for planting from all countries (excluding seed).
Although a less aggressive form of P. alni is found in Alaska, we can reduce its spread. The more aggressive form found in Europe is causing disruptions to stream and river ecosystems. Vigilance on our part can reduce the chances of similar damage in Alaska.
Reporting and submitting samples
Early Detection and Rapid Response (EDRR) is a framework used by federal and state agencies to manage exotic or invasive pests. The goals are detection of pests before they can establish, assessment of management options, and quick response to prevent spread and minimize damage and expense. If you find a disease you don’t recognize as native, submit a sample to a laboratory for diagnosis.
- Call the lab to ensure they can perform the tests needed. This also gives them lead time to prepare for your samples and expect their arrival. Call your local UAF Cooperative Extension Office, and fill out an identification form; or call the Alaska Division of Agriculture, Plant Materials Center, Plant Pathology Laboratory: (907) 745-8138, http://plants.alaska.gov/PathologyForms.html.
- Examine the plant carefully; damage further down may cause symptoms higher on the plant.
- If possible, include a healthy example, as well as various stages of disease development from early to more severe.
- Keep samples cool, not frozen.
- Send entire plants if possible, including roots. Place roots in a plastic bag, and rubber band to keep soil from damaging or obscuring other symptoms on plant.
- Fleshy specimens (such as potatoes or fruit) should be as firm as possible, with early to intermediate symptoms. Place in paper bags (they will rot in plastic bags). Place wrapped samples in plastic zipper-top bags. Place the plastic bag inside another plastic zipper-top bag (this gives a double-bagged sample for security). Pack box with sufficient newspaper or paper towels to absorb any leaks, and to protect samples.
- Wrap other sample types in dry paper towels or newspaper. Do not add moisture, as samples can rot in transit. Place wrapped samples in plastic zipper-top bags. Place the plastic bag inside another plastic zipper-top bag (this gives a double-bagged sample for security). Pack remainder of box with newspaper to protect samples.
- Collect specimens as close to mailing as possible, keep cool, and ship by fastest method available. Ship early in the week (Mon.-Wed.) to ensure it arrives before the weekend. Delays can result in samples rotting, or being overgrown with other organisms; this may make accurate diagnosis difficult or impossible.
- If sending to the Alaska Division of Agriculture, the address is:
Plant Pathology Laboratory
Alaska Plant Materials Center
5310 S. Bodenburg Spur Rd.
Palmer, AK 99645
- Digital photos may also be submitted to aid in diagnosis: todd.steinlage@alaska.gov
Sources and More Information
APHIS List of Regulated Hosts and Plants Proven or Associated with Phytophthora ramorum. 2013.
Working Group for Phytophthoras in Native Habitats. 2016. Guidelines to Minimize Phytophthora Pathogens in Restoration Nurseries.
Sullivan, M. 2010. CPHST Pest Datasheet for Phytophthora alni. USDA-APHIS-PPQ-CPHST.
Adams, G.C., Catal, M., and Trummer, L. 2009. Distribution and Severity of Alder Phytophthora in Alaska. Proceedings of the Sudden Oak Death Fourth Science Symposium. June 15-18, 2009, Santa Cruz, California. Pp. 29-49.
Federal Order for Phytophthora alni. DA-2009-27.
Bjelke, U., Boberg, J., Oliva, J., Tattersdill, K., and McKie, B. 2016. Review: Dieback of riparian alder caused by the Phytophthora alni complex: projected consequences for stream ecosystems. Freshwater Biology 61: 565–579.
Adams, G. C., Catal, M., Trummer, L., Hansen, E. M., Reeser, P., and Worrall, J. J. 2008. Phytophthora alni subsp. uniformis found in Alaska beneath thinleaf alders. Online. Plant Health Progress. DOI:10.1094/PHP-2008-1212-02-BR.
Davidson, J. M., Werres, S., Garbelotto, M., Hansen, E. M., and Rizzo, D. M. 2003. Sudden oak death and associated diseases caused by Phytophthora ramorum. Online. Plant Health Progress. DOI:10.1094/PHP-2003-0707-01-DG.
Federal Order for Restrictions on importation of Phytophthora ramorum (P. ramorum) host plants for planting into the United States. DA-2012-14.
Shishkoff, N. 2014. Growth-inhibiting fungicides affect detection of Phytophthora ramorum from infected foliage and roots. Online. Plant Health Progress. DOI:10.1094/PHP-RS-12-0124.
Shishkoff, N. 2009. Propagule production by Phytophthora ramorum on lilac (Syringa vulgaris) leaf tissue left on the surface of potting mix in nursery pots. Plant Disease. 93:475-480.
Shishkoff, N. 2007. Persistence of Phytophthora ramorum in soil mix and roots of nursery ornamentals. Plant Disease. 91:1245-1249.
Shishkoff, N. 2012. Susceptibility of some common container weeds to Phytophthora ramorum. Plant Disease. 96:1026-1032.
Jones, B., and Wylder, B. 2012. Phytophthora ramorum: Identifying symptomatic larch. UK Forestry Commission.
National Pest Alert: Sudden Oak Death: Phytophthora ramorum USDA–CSREES Integrated Pest Management Centers
Tjosvold, S.A., Buermeyer, K.R., Blomquist, C., and Frankel, S. 2005. Nursery Guide for Diseases Caused by Phytophthora ramorum on Ornamentals: Diagnosis and Management. University of California Publication 8156.
Grunwald, N.J., Garbelotto, M., Goss, E.M., Heungens, K., and Prospero, S. 2012. Review: Emergence of the sudden oak death pathogen Phytophthora ramorum. Trends in Microbiology 20(3): 131-138. DOI:10.1016/j.tim.2011.12.006
Grunwald, N.J., Goss, E.M., and Press, C.M. 2008. Pathogen profile Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Molecular Plant Pathology 9(6): 729-740. DOI:10.1111/J.1364-3703.2008.00500.X
Aguayo, J., Adams, G.C., Halkett, F., Catal, M., Husson, C., Nagy, Z.Á., Hansen, E.M., Marçais, B., and Frey, P. 2013. Strong genetic differentiation between North American and European populations of Phytophthora alni subsp. uniformis. Phytopathology 103:190-199.