Learning Objectives
- What fungi are, and how they damage plants
- Symptoms of fungal diseases
- Potential exotic disease threats to Alaska
- How to report or submit samples for diagnosis
- Vocabulary associated with fungal diseases
Fungi are an ancient group of very diverse organisms including yeasts, mushrooms, lichens, plant and animal parasites, and molds. Over 100,000 species have been described, with many more yet to be discovered. The earliest known fungal fossils date to 460-455 million years old; DNA evidence indicate they may have arisen over 1 billion years ago. Fungi were once considered primitive plants, but are now seen as more closely related to animals.
Most fungi have mycelium made of threadlike hyphae. Most of the mycelium is often hidden in the food substrate. Many fungi also produce fruiting bodies, which contain spores. Spores are used for survival, reproduction, and dispersal. Food is digested externally by use of enzymes, and nutrients are absorbed directly. Some fungi obtain nutrients from dead organic matter, they are very important in decomposition and recycling of nutrients.

Other fungi obtain nutrients from living matter. Relationships with hosts are on a spectrum from mutualism (both benefit), to commensalism (one benefits without significant effect on the other), to parasitism (parasites benefit at the expense of the host). These relationships may change with environmental conditions, host development, or physiology. Examples of mutualism include mycorrhizal fungi (aiding in nutrient uptake) and endophytic fungi (protection from herbivores and aid survival in extreme environments). Commensalism may be demonstrated by fungi colonizing a non-host leaf, without causing disease.
|
|
|
Most known plant diseases are caused by fungi; thousands of fungal species are capable of causing disease in plants. Diseases caused by fungi include seedling damping off, wilts, leaf spots, cankers, galls, and rots. In addition to disease in plants, some plant-infecting fungi also produce toxins with health impacts on humans and other animals. Examples include ergots of grasses and mycotoxins produced in grains and nuts.
Exotic Fungal Pathogens
Brown rot of fruit (caused by Monilinia fructigena and Monilia polystroma)
Monilinia fructigena, and Monilia polystroma are closely related fungi, and are among those responsible for brown rot in fruit trees. They primarily cause disease in pome fruits (apples and pear) and stone fruits (apricot, plum, peach and cherry). M. fructigena is currently found in the Mediterranean basin of Africa, throughout Asia, and throughout Europe. It was discovered in Maryland in 1979 and eradicated. It has also been found twice on fruit from Mexico, but trace-backs were unable to find an infected source. In Europe, most losses are in the field (<10% to over 40%), with an additional few percent lost in storage. The greatest losses are seen in apple and plum. M. polystroma has been found in China, Japan, and parts of Europe. Neither is currently found in the U.S.

Hosts and Symptoms
Hosts of M. fructigena and M. polystroma include apple, pear, Prunus spp. (apricot, cherry, nectarine, peach, and plum), strawberry, cotoneaster, hawthorn, rhododendron, rose, tomato, and blueberry. Both fungi are favored by moist conditions (rain, fog, dew, etc.), especially at the beginning of the season. They are rarely seen in arid production areas. The fungi overwinter in mummified fruit still on the tree, as well as fallen fruit; they also survive in branch cankers.
Spores are produced in the spring, and easily spread by wind, water, insects, birds, and humans. They do not require liquid water for spore germination. Spores may infect flowers, causing blossom blight or twig blight. Brown spots may form on flowers and twigs. Flowers may wilt and collapse, some remaining on the tree and others falling. Twigs may die as the fungus moves toward larger branches. Moist conditions with moderate temperatures cause spore production and multiple cycles of infection may occur.
|
|
|
Cankers may develop on blighted twigs or branches. They are generally elliptical (elongated circles) or spindle shaped (larger in the middle, tapered at ends). Gum may be produced at the leading edges.

If the stem is girdled, leaves beyond the canker may be blighted. Leaves on infected twigs may be dark brown and remain attached. Cankers are usually on twigs and don’t extend into previous year’s wood. Additional spores may be produced on canker surfaces. Unlike many other canker diseases, those caused by M. fructigena and M. polystroma do not continue to enlarge year after year. If the branch is not girdled, callus (wound healing) tissue will form around the canker margins. Other fungi and bacteria may invade cankers and dead shoots.
The most important disease phase is brown rot of fruit. Infections take place via cracks and wounds (including scab lesions, growth cracks, hail damage, and insect damage), as well as by fruit-to-fruit contact. Fruit may be infected at any stage of development. Infections may be inactive until conditions favor disease development. Fruit may also become infected during harvest or storage. Initial lesions are small, brown, circular, and firm. These spots rapidly expand and may rot the entire fruit.

When the relative humidity is high and fruit are ripe or nearly ripe, tufts of cream-white to tan mycelia and spores may erupt from the skin. These often form concentric rings.
Rotted fruit may drop or stay attached to the tree, eventually drying into hard shriveled mummies. Fruit in storage may rot, apples may turn brown or black. Fruit rot is common in apple, pear, and plum. Rot is less severe in apricot, nectarine, and peach. M. polystroma may colonize fruit faster than M. fructigena; it also produces a larger fungal mass, which may inhibit decomposition, and may increase the amount of inoculum the following spring. Some infected fruit show no symptoms.
Similar symptoms may be caused by other common fungi. Related fungi, Monilinia fructicola and Monilinia laxa are widespread in the U.S., their pustules are grayish-brown (as opposed to the light yellowish-brown of M. fructigena and M. polystroma). Penicillium spp. and Mucor spp. can also cause a fruit rot similar to M. fructigena.
The main pathways of introduction to new areas are infected fruit and vegetative propagation material. USDA-APHIS conducted a host risk analysis for the contiguous U.S. based on the presence of susceptible hosts. The eastern U.S. has a high risk of pathogen establishment while they found California, Oregon, and Washington to have moderate risk of establishment. Though Alaska was not included in the analysis, the risk is probably similar to, or lower than, Washington due to lack of large orchards. However, the climate of much of Alaska is conducive to pathogen survival and spread.
Potato wart (caused by Synchytrium endobioticum)
Synchytrium endobioticum is a fungal pathogen of potato, causing potato wart. Potato wart is a devastating disease, making tubers unmarketable, and causing up to 100% losses. Only potato and wild Solanum spp. are infected. Potato wart is found throughout Africa, Asia, Europe, Canada (Newfoundland and Prince Edward Island), South America, and New Zealand. When S. endobioticum was found on Prince Edward Island in 2000, the overall losses and cost of containment was $83 million. It has been eradicated from Maryland, Pennsylvania, and West Virginia. It is not currently found in the U.S.
S. endobioticum is an obligate parasite meaning it must feed on living tissue. Interestingly, it does not produce hyphae, as most fungi do. It produces sporangia, full of 200-300 swimming zoospores. Winter (resting) sporangia are thick-walled and resistant to physical and chemical degradation. When spring temperatures rise above 46°F and moisture is sufficient, resting sporangia germinate, releasing zoospores. Zoospores move through soil water to find potato hosts. S. endobioticum does not infect roots. Infections occur on buds, stolon tips, and leaf primordia (future leaves) of tubers and the lower stem. The optimum temperature for infection is 54-75°F. Cells become enlarged and undergo rapid cell division. Thin-walled summer sporangia are formed which release more zoospores in multiple infection cycles. The rapid growth of tissue creates breaks in the surface and opens the plant to additional infections. When conditions become unfavorable (ex. dry), winter (resting) sporangia are formed. These sporangia are very durable, and can remain viable for 40 years. They can survive down to at least 19” soil depth.

Potato wart only affects potato and wild Solanum spp. Symptoms are most severe under cool, wet conditions. Plants are not killed, but sprout emergence may be reduced, and general plant vigor suffers. Potato wart doesn’t usually cause aboveground symptoms, but stems, leaves, and flowers can develop galls. Stem bases may show small, greenish warts. Stolons (underground stems) and tubers are the main points of infection. Infection of young tubers causes distortion and sponginess. Older tubers are usually infected at the eyes. Young warts are white, soft and pulpy, with rough corrugated surfaces. Warts may be green if exposed to light. Size varies from ¼” to 3” diameter. With time, they gradually darken, and may dry or rot. Warts may continue developing in storage. They can be entry points for other organisms. Symptoms could be confused with powdery scab (superficial, powdery lesions), or potato smut (white inside, with brown cavities). None of these diseases (potato wart, powdery scab, or potato smut) are known to occur in Alaska, submit suspect samples to a laboratory for identification.
|
|
|
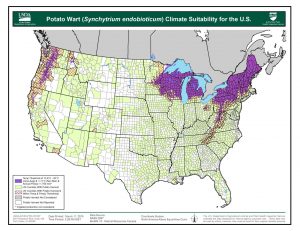
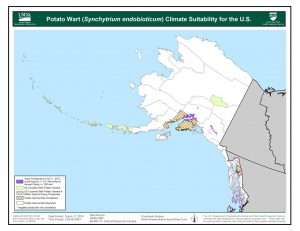
S. endobioticum grows best in areas with low average summer temperatures (less than 64°F), long winters (greater than 160 days with temperatures below 41°F), and precipitation greater than 700mm (27.5”), mostly in summer. Short potato rotations (potatoes every 2-3 years) and cultivated soils also favor the pathogen. Much of the northern U.S. and Canadian potato production areas have climates suitable for S. endobioticum. This includes much of the Pacific Northwest, Great Lakes, and Northeast. Southeast Alaska, the Kenai Borough, and the Matanuska-Susitna Borough have suitable areas.
|
|
|
S. endobioticum is introduced to new areas by movement of tubers, soil, machinery, and footwear. Irrigation water and wind may also carry sporangia. Sporangia survive the digestive tracts of animals, and can be spread by manure. Composting is insufficient to kill sporangia. Chemical soil treatments are not a viable option for eradication. Resistant potato varieties exist, but resistance-breaking isolates of S. endobioticum have been found. In potato varieties with partial resistance, warts are superficial and scab-like.
Phytosanitary measures, including quarantines, restrictions, and sanitation practices, have helped to contain potato wart. Under the European Union Wart Control Directive, infested fields are banned from potato cultivation for 20 years, and adjacent areas also face planting restrictions. A U.S. Federal Order in 2015 addressed requirements for potatoes imported from Prince Edward Island, Canada (DA-2015-01, see in references). Conditions require inspection of tubers, phytosanitary certificates, and may require soil testing (based on the end use of the potatoes).
For a printable version of information on potato wart view and download our factsheet here.
Reporting and submitting samples
Early Detection and Rapid Response (EDRR) is a framework used by federal and state agencies to manage exotic or invasive pests. The goals are detection of pests before they can establish, assessment of management options, and quick response to prevent spread and minimize damage and expense. If you find a disease you don’t recognize as native, submit a sample to a laboratory for diagnosis.
- Call the lab to ensure they can perform the tests needed. This also gives them lead time to prepare for your samples and expect their arrival. Call your local UAF Cooperative Extension Office, and fill out an identification form; or call the Alaska Division of Agriculture, Plant Materials Center, Plant Pathology Laboratory: (907) 745-8138, http://plants.alaska.gov/PathologyForms.html.
- Examine the plant carefully; damage further down may cause symptoms higher on the plant.
- If possible, include a healthy example, as well as various stages of disease development from early to more severe.
- Keep samples cool, not frozen.
- Send entire plants if possible, including roots. Place roots in a plastic bag, and rubber band to keep soil from damaging or obscuring other symptoms on plant.
- Fleshy specimens (such as potatoes or fruit) should be as firm as possible, with early to intermediate symptoms. Place in paper bags (they will rot in plastic bags). Place wrapped samples in plastic zipper-top bags. Place the plastic bag inside another plastic zipper-top bag (this gives a double-bagged sample for security). Pack box with sufficient newspaper or paper towels to absorb any leaks, and to protect samples.
- Wrap other sample types in dry paper towels or newspaper. Do not add moisture, as samples can rot in transit. Place wrapped samples in plastic zipper-top bags. Place the plastic bag inside another plastic zipper-top bag (this gives a double-bagged sample for security). Pack remainder of box with newspaper to protect samples.
- Collect specimens as close to mailing as possible, keep cool, and ship by fastest method available. Ship early in the week (Mon.-Wed.) to ensure it arrives before the weekend. Delays can result in samples rotting, or being overgrown with other organisms; this may make accurate diagnosis difficult or impossible.
- If sending to the Alaska Division of Agriculture, the address is:
Plant Pathology Laboratory
Alaska Plant Materials Center
5310 S. Bodenburg Spur Rd.
Palmer, AK 99645
- Digital photos may also be submitted to aid in diagnosis: todd.steinlage@alaska.gov
Sources and More Information
Carris, L.M., Little, C.R., and Stiles, C.M. 2012. Introduction to Fungi. The Plant Health Instructor. DOI:10.1094/PHI-I-2012-0426-01
Redman, R.S., Dunigan, D., and Rodriguez, R.J. 2001. Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? Papers in Plant Pathology. 104. https://digitalcommons.unl.edu/plantpathpapers/104
Sullivan, M., and Mackesy, D. 2013. CPHST Pest Datasheet for Monilinia fructigena. USDA-APHIS-PPQ-CPHST.
Sullivan, M. 2013. CPHST Pest Datasheet for Monilia polystroma. USDA-APHIS-PPQ-CPHST.
Chalkley, D. Systematic Mycology and Microbiology Laboratory, ARS, USDA. Invasive Fungi. Asian/European brown rot of Rosaceae – Monilinia fructigena. Retrieved January 2, 2018, from /sbmlweb/fungi/index.cfm
Molet. T., Mackesy, D., and Sullivan, M. 2014. CPHST Pest Datasheet for Synchytrium endobioticum. USDA-APHIS-PPQ-CPHST.
Obidiegwu, J.E., Flath, K., and Gebhardt, C. 2014. Managing potato wart: a review of present research status and future perspective. Theoretical and Applied Genetics 127:763–780. DOI:10.1007/s00122-014-2268-0
CABI Datasheet for Synchytrium endobioticum https://www.cabi.org/isc/datasheet/52315
Federal Order for U.S. Imports of Potatoes from Prince Edward Island (PEI), Canada to Prevent the Entry or Introduction of Synchytrium endobioticum, the Causative Agent of Potato Wart. DA-2015-01