Learning Objectives
- What bacteria and phytoplasmas are, and how they damage plants
- Symptoms of bacteria and phytoplasma diseases
- Potential exotic disease threats to Alaska
- How to report or submit samples for diagnosis
- Vocabulary associated with bacteria and phytoplasma diseases
Bacteria
Bacteria are tiny, single-celled organisms. They range in size from 0.1- 10µm (micrometer= millionth of a meter). They are an ancient group, with fossil evidence from 3.5 billion years ago. Bacteria are very diverse, living in many environments, on many food sources. They are important components of food webs, recycling nutrients, as well as providing useful products such as antibiotics and insulin. Many bacteria are aerobic (require oxygen), others are anaerobic (use other substances for respiration), and still others are facultative (capable of using oxygen or other metabolic pathways). Some bacteria are pathogenic to living organisms. Many bacteria are capable of very rapid growth under the proper environmental conditions.
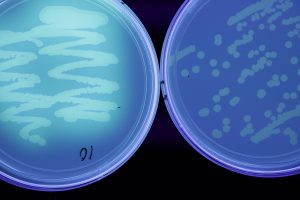
Some bacteria can form endospores, tough structures to help them survive high temperatures, UV light, desiccation (drying), chemicals or enzyme degradation. They may also form biofilms on surfaces, these are dense growths of multiple layers, protecting the bacteria from environmental stresses. Spores and biofilms may allow survival for many years. Bacteria also survive in plant debris, soil, water, in and on seed, perennial host plants, insect hosts, machinery, or clothing. Many pathogenic bacteria are capable of living on dead organic matter, and are opportunistic pathogens. Entry to plants may occur through seed, wounds, lenticels or stomata (gas exchange pores), or hydathodes (water exuding pores on leaf edges). Bacteria are often spread by wind and rain splash, infected plant material, insects, humans and machinery.
Symptoms of bacterial infections vary widely. Bacteria may cause mosaic patterns (light and dark patches), spots on leaves or fruit, blight (collapse of leaves), wilting, galls, distortions, and rots.
Bacteria are managed through certification and clean plant programs. Removal and destruction of diseased tissue reduces available inoculum (those parts able to cause infection). Crop rotation to non-host crops allows for bacterial populations to decline. Plant breeders have introduced varieties with at least partial resistance to many important pathogens. Sanitation and chemical pesticides can be very useful in management of bacterial pathogens, as well as cultural options such as irrigation management.
Exotic Bacterial Pathogens
Brown rot, Southern wilt, and Bacterial wilt (caused by Ralstonia solanacearum)
Ralstonia solanacearum is a bacterium, causing bacterial wilt in over 200 plant species. There are multiple races, which are based on host ranges. Within races, there are multiple “biovars”, which are differentiated by their nutrient requirements. Many bacterial wilt diseases occur in tropical and subtropical lowlands. Ralstonia solanacearum Race 3, biovar 2 (R3bv2) is of particular concern, as it can cause disease at higher altitudes or in temperate zones, at lower temperatures. R3bv2 is currently found in Africa, Asia, Australia, Europe, South America, and central America (including Mexico). It has been eradicated in the U.S. and Canada multiple times. Worldwide, R3bv2 causes an estimated $1 billion in losses every year. There are other races of R. solanacearum currently found in the U.S.; diagnostic testing is required to identify the specific race and biovar.
Hosts and Symptoms
R3bv2 causes brown rot in potato, Southern wilt in geranium, and bacterial wilt in tomato and eggplant. Weed hosts include: climbing nightshade, stinging nettles, portulaca, pigweed, and chickweed. Infected hosts may not show symptoms, even with high bacterial loads. Little symptom development occurs at temperatures below 61°F, symptoms are most severe from 75-95°F. Symptoms in any host are not sufficient to differentiate R3bv2 from other Ralstonia solanacearum isolates found in the U.S.
In potato, symptoms can occur at any stage of growth. Young potato stems may show dark brown, narrow streaks in their vascular tissue; this may be visible through the epidermis, or after longitudinal or cross-sectioning.
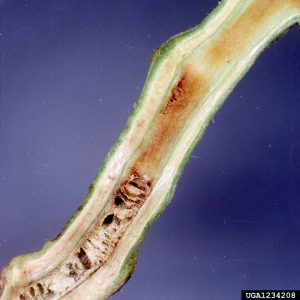
Symptoms may occur in isolated stems, branches, or one side of leaflets. Leaves (especially young) may curl upward or wilt and foliage may yellow. Plants can wilt quickly; leaves may wilt while green and dry on the plant. Entire plants may be stunted (short) or killed.

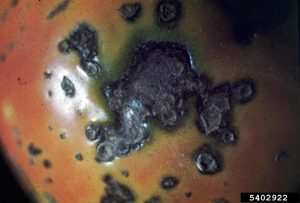
Potato tubers may exhibit grayish-brown eyes at the bud end. A sticky exudate may be present at the bud or stolon end, soil may adhere. Cross-sections or longitudinal cuts of tubers may show a grayish-brown discoloration of the vascular ring (xylem and phloem), this may extend inward to the pith, or outward to the cortex.
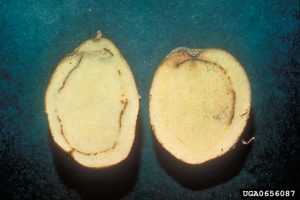
Grayish-white droplets of bacterial ooze may emerge from the vascular ring with light pressure on the tuber. If cut tuber halves are pressed together and slowly pulled apart, fine bacterial threads may be visible. Cross-sectioned stems may show glistening gray droplets of bacterial ooze. Suspending this stem in water, and waiting 15 minutes may show fine milky-white strands of bacteria.

Symptoms may be mistaken for bacterial ring rot (Clavibacter michiganensis subsp. sepedonicus), blackleg (Erwinia and Dickeya spp.), Fusarium spp., Verticillium spp., drought, root damage, or nutrient deficiencies. Tomato and eggplant may show similar symptoms to potato, or may develop symptoms just before ripening and then collapse.
Symptoms in geraniums are most strongly expressed at 84-95°F. A characteristic symptom is wilting of lower leaves, becoming chlorotic (yellowing); this progresses to upward curling of leaf margins.

Wilted leaves may recover at night. Wilted leaves may develop wedge-shaped areas of chlorosis, which may become necrotic. Leaf margins may also become chlorotic, turning necrotic. Stems may collapse, whole plants may desiccate and die. Root crowns, roots and stems may show vascular blackening and necrosis. As with potato and tomato, cut stems in water may show bacterial streaming. Geraniums are often symptomless, though they may harbor large bacterial populations.
Symptoms of bacterial wilt in geranium are similar to bacterial blight (Xanthomonas campestris pv. pelargonii), however bacterial blight also causes tan/brown, round leaf spots.

R. solanacearum is primarily soilborne and waterborne, it does not spread aerially. Growing roots produce small wounds as lateral roots emerge, bacteria can enter these wounds, as well as those caused by insects or other injury. R. solanacearum can survive in infected soil or water up to two years after hosts have been removed. Contaminated soil and water easily spread bacteria between plants, and can serve as pathways of introduction to new areas. Infected plants, cuttings, and tubers are responsible for worldwide spread; even asymptomatic plants can shed large numbers of bacteria. Hanging baskets have dripped bacteria onto plants below them. Contaminated pots, tools, benches, and equipment also play important roles in spread.
For a printable version of information on bacterial wilt view and download our factsheet here.
Phytoplasmas
Phytoplasmas are single-celled organisms descended from bacteria, but they have lost the cell wall. They are considered mollicutes (“soft skin”), and able to change shape in response to their environment. Phytoplasmas are obligate parasites that only live in the phloem cells of plants or in their insect vectors. They cannot be grown in pure culture (many bacteria can be grown in culture). Due to the inability to grow them without a host, phytoplasmas are not given Latin names (genus and species). Instead, they are referred to as ‘Candidatus Phytoplasma’.
Phytoplasmas are transmitted by leafhoppers, planthoppers, or psyllids; they reproduce in their vectors. Over 1000 plant species are known to be infected by phytoplasmas, including: apples, cherries, potatoes, tomatoes, strawberries, and clovers. Phytoplasma diseases were assumed to be caused by viruses until the late 1960’s, when newer detection capabilities were developed.
Symptoms of phytoplasma infections include two main types: yellows and witches’ brooms. Yellows symptoms are due to phloem dysfunction and changes to resource allocation. Plants may have a general unhealthiness, foliage may appear chlorotic (yellow), wilting may occur, stunting of plants, dieback, and necrosis (death) of phloem may occur.

Witches’ brooms are due to growth regulator changes. Symptoms include proliferation of axillary shoots, bunchy top growth, swollen veins, asymmetrical flowers, sterile flowers, abnormal petal color breaking (dark and light colors), phyllody (leafy structures in place of normal flower parts), or virescence (green petals).
Management of phytoplasmas is accomplished by planting clean materials. Phytoplasmas are resistant to antibiotics that target cell walls (which they don’t have), but other antibiotics may be used for especially valuable plants. Heat therapy and meristem culture may be used to clean germlines. Monitoring of insect vectors and application of insecticides may reduce the spread of phytoplasmas.
Exotic Phytoplasma Pathogens
Apple proliferation (caused by Phytoplasma mali)
‘Candidatus Phytoplasma mali’ is the cause of apple proliferation (AP). The AP phytoplasma is found throughout Europe (eradicated in the U.K.), Tunisia, Syria, and Turkey. There are unreliable reports of its presence in India, Russia, and South Africa. There has also been an unconfirmed report from Canada. In apples, AP phytoplasma may cause up to 50% reduction in fruit size, reduced sugar and acidity, reduced vigor and tree growth, and may kill young trees. Due to quality reduction, the value of fruit may decrease 30-100%. Trees infected with AP phytoplasma are more susceptible to powdery mildew.
Psyllids (Cacopsylla picta, and C. melanoneura) and leafhoppers (Fieberiella florii) transmit AP phytoplasma. The insects transmit in a persistent, propagative manner (i.e. they are infective for life, and the phytoplasma multiplies in the insect). Psyllids usually overwinter as adults on conifers, where they remain infective. They migrate from overwintering hosts to apples, then reproduce. Both nymphs and adult psyllids transmit AP phytoplasma. It is unknown if any of the vectors are present in Alaska, but psyllids of the genus Cacopsylla have been recorded in Alaska. Fieberiella florii overwinters as eggs and adults on boxwood, Cotoneaster, and Malus spp. (apples and crabapples); it has been documented in British Columbia and Ontario.
|
|
|
Phytoplasmas are graft transmissible, and often disseminated in scion wood. Transmission may also occur through root grafts in apple orchards. There are no reports of seed or pollen transmission.
Phytoplasma concentrations in aerial portions of the tree decrease in winter. Concentrations increase in spring, growing to their highest levels in late summer and early fall. Aerial parts of the tree are not evenly colonized. The AP phytoplasma is detectable in roots year-round. Mild temperatures of 70-77°F encourage spread throughout the tree, while higher temperatures of 84-90°F inhibit symptom development (and the phytoplasma may only be found in roots). Plants may undergo “recovery”, a spontaneous remission of symptoms, but remains infected. Scions removed from infected plants during dormancy have reduced phytoplasma transmission; those removed during summer have much higher transmission.
Hosts and Symptoms
Apple proliferation is a disease of apple, cherry, apricot, plum, dahlia, rose, lily, forsythia, viburnum, and spiraea. In apple, infected plants lack vigor and show reduced growth, often with thin shoots. Dense clusters of secondary shoots (“witches’ brooms”) may form, especially at the apex of the main shoot (normal lateral branches are near the base of the shoot). Witches’ brooms can appear on different parts of the tree each year, usually developing only during the first 2-3 years after infection.

Normal dormant buds may be replaced by a rosette of terminal leaves, these are often infected by powdery mildew. Shoot tips may die. Leaves appear earlier in the spring than normal, posing risk for cold damage. Leaves may roll downward, becoming brittle. Leaves may be smaller than normal, with fine and irregular serrations. Leaves may show chlorosis. Petioles are shortened, and stipules lengthened (stipules are small leaves at the base of petioles).

Flowering may be delayed (often only on part of the tree) until late summer or fall. Flowers may produce numerous petals or appear asymmetrical. Fruit may fail to set or drop prematurely. Fruit may appear flattened, with the calyx (flower) end shallow and broad. Fruit may be reduced in size, with incomplete coloration and poor flavor. Seeds may be small. Bark may be fluted lengthwise (an incurving of bark) with a reddish-brown color. Necrotic areas may appear on the bark. Branches may die. In fall, terminal buds may set late. Root weight is reduced, with fibrous roots forming compact, felt-like masses. Infected trees may occur in clusters, expanding year to year. Some phytoplasma strains cause very severe symptoms, whereas others may cause mild symptoms. Plants can be infected with multiple phytoplasma strains at the same time.
| Host | Symptoms |
| Cherry | Wilting, floral and vascular necrosis |
| Apricot | Stem necrosis, leaf wilting |
| Plum | Late blooming |
| Dahlia | Bushy growth, witches’ brooms, narrow leaves, few flower buds |
| Rose | Dieback, witches’ brooms, bud proliferation, stunted growth, leaf and flower malformation |
| Lily | Leaf scorch, leaf malformation and necrosis, flower buds may drop off |
| Forsythia | Leaf reddening |
| Viburnum | Witches’ brooms |
| Spiraea | General decline |
Table 1. Native and Domesticated AP phytoplasma Hosts in Alaska
For a printable version of information on apple proliferation view and download our factsheet here.
European stone fruit yellows (caused by Phytoplasma prunorum)
‘Candidatus Phytoplasma prunorum’, is the cause of European stone fruit yellows (ESFY). Other common names include peach decline, peach yellows, peach vein-clearing, and apricot dieback. The ESFY phytoplasma is found throughout Europe, north Africa, and western Asia. Though related to the apple proliferation phytoplasma (AP), it is a distinct organism. It is also separate from the North American “X-diseases”, also caused by phytoplasmas.
The primary insect vector of ESFY phytoplasma is the plum psyllid (Cacopsylla pruni). Plum psyllids overwinter as adults on conifers, moving to Prunus spp. to reproduce. They only feed on Prunus, and only produce one generation per year. New adults leave as soon as they are developed, to fly to their overwintering host. Their preferred summer hosts, blackthorn (Prunus spinosa) and mahaleb cherry (Prunus mahaleb) are not reported in Alaska, but they are found in British Columbia. Psyllids are infected for life, and the phytoplasma multiplies in the insect. Psyllids require 1-2 days of feeding to transmit the ESFY phytoplasma. The specific vector species has not been reported from Alaska, but many other Cacopsylla species have been, along with several specimens identified only to genus level. Other possible vectors include leafhoppers in the genera Euscelis and Empoasca, both of which have member species in Alaska.
|
|
|
Trees may not show symptoms for 4-5 months after infection. In contrast to the AP phytoplasma, ESFY phytoplasma can persist in Prunus stems during winter dormancy. It is graft transmissible, and capable of rapid spread within individual plants, as well as between plants.
Hosts and Symptoms
In general, ESFY symptoms are strongly influenced by host species, cultivar, rootstock, environmental conditions, and phytoplasma isolate. Often, only a few branches show symptoms, but the whole tree may be affected. Leaves are often chlorotic late in the season. Shoots may be shortened, with small deformed leaves. Premature leaf drop may occur. Fruit production is reduced, with poor development, and may drop early. In late winter, leaf buds may break early, increasing the risk of frost damage. In sensitive varieties, young trees may die within 1-2 years, though older plants are also susceptible.
In apricots and Japanese plum, leaves become chlorotic, rolling upward longitudinally (along the long axis), followed by reddening. Normal dormancy is reduced or suppressed. Severe and progressive necrosis occurs, followed by decline and possible death of the tree.
|
|
|
In peach, leaves may show severe longitudinal upward rolling, with early reddening. Midribs and primary veins may become thickened and corky. Leaves may become brittle, and fall early. In the fall, latent buds may form, producing tiny chlorotic leaves and sometimes flowers.
Other hosts include multiple species of cherry, including European bird cherry. Cherry trees may bloom abundantly, flowers are malformed with poor fruit set. Fruit are small with short peduncles and drop prematurely. Leaves are small and deformed with rosetting (shortened internodes), as well as phloem and bark necrosis.
Similar symptoms can be caused by Phytophthora root rot, which can cause yellowing and stunting, though generally all branches are affected unlike ESFY, which doesn’t usually affect all branches. Digging up the roots of a Phytophthora-infected tree will show red to brown rotting of the root system, with sharp lines separating healthy and diseased tissue. ESFY has some symptoms in common with the North American “X-diseases” , also caused by phytoplasmas. Leaves may yellow, roll, and veins may swell. X-diseases also cause yellow or red, irregular shaped leaf spots. These spots appear water-soaked, later drying and dropping out. Proper diagnosis requires laboratory testing.
|
|
|
Most of the continental U.S. has a fairly low risk for establishment of ESFY phytoplasma, based on host availability. Due to Alaska’s low density of susceptible hosts, the risk of establishment is expected to be low.
Long distance dispersal of the ESFY phytoplasma is by planting materials (plants, scions, and rootstock).
Suspected phytoplasma samples of symptomatic leaf tissue should be collected in the late summer or early fall, when phytoplasma concentrations are at their highest. At least 5 samples per plant should be taken from different parts of the canopy.
Reporting and submitting
Early Detection and Rapid Response (EDRR) is a framework used by federal and state agencies to manage exotic or invasive pests. The goals are detection of pests before they can establish, assessment of management options, and quick response to prevent spread and minimize damage and expense. If you find a disease you don’t recognize as native, submit a sample to a laboratory for diagnosis.
- Call the lab to ensure they can perform the tests needed. This also gives them lead time to prepare for your samples and expect their arrival. Call your local UAF Cooperative Extension Office, and fill out an identification form; or call the Alaska Division of Agriculture, Plant Materials Center, Plant Pathology Laboratory: (907) 745-8138, http://plants.alaska.gov/PathologyForms.html.
- Examine the plant carefully; damage further down may cause symptoms higher on the plant.
- If possible, include a healthy example, as well as various stages of disease development from early to more severe.
- Keep samples cool, not frozen.
- Send entire plants if possible, including roots. Place roots in a plastic bag, and rubber band to keep soil from damaging or obscuring other symptoms on plant.
- Fleshy specimens (such as potatoes or fruit) should be as firm as possible, with early to intermediate symptoms. Place in paper bags (they will rot in plastic bags). Place wrapped samples in plastic zipper-top bags. Place the plastic bag inside another plastic zipper-top bag (this gives a double-bagged sample for security). Pack box with sufficient newspaper or paper towels to absorb any leaks, and to protect samples.
- Wrap other sample types in dry paper towels or newspaper. Do not add moisture, as samples can rot in transit. Place wrapped samples in plastic zipper-top bags. Place the plastic bag inside another plastic zipper-top bag (this gives a double-bagged sample for security). Pack remainder of box with newspaper to protect samples.
- Collect specimens as close to mailing as possible, keep cool, and ship by fastest method available. Ship early in the week (Mon.-Wed.) to ensure it arrives before the weekend. Delays can result in samples rotting, or being overgrown with other organisms; this may make accurate diagnosis difficult or impossible.
- If sending to the Alaska Division of Agriculture, the address is:
Plant Pathology Laboratory
Alaska Plant Materials Center
5310 S. Bodenburg Spur Rd.
Palmer, AK 99645
- Digital photos may also be submitted to aid in diagnosis: todd.steinlage@alaska.gov
Sources and More Information
Vidaver, A.K. and P.A. Lambrecht 2004. Bacteria as plant pathogens. The Plant Health Instructor. DOI: 10.1094/PHI-I-2004-0809-01
Fletcher, J. and A. Wayadande. 2002. Fastidious vascular-colonizing bacteria. The Plant Health Instructor. DOI: 10.1094/PHI-I-2002-1218-02
Sullivan, M., Daniells, E., Southwick, C., and Mackesy, D. 2013. CPHST Pest Datasheet for Ralstonia solanacearum race 3 biovar 2. USDA-APHIS-PPQ-CPHST.
National Pest Alert: Ralstonia solanacearum, race 3, biovar 2. USDA–CSRESS Integrated Pest Management Centers.
Champoiseau, P. G., Jones, J. B., and Allen, C. 2009. Ralstonia solanacearum race 3 biovar 2 causes tropical losses and temperate anxieties. Online. Plant Health Progress DOI:10.1094/PHP-2009-0313-01-RV.
Seemüller, E., and B. Schneider. 2004. ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. International Journal of Systematic and Evolutionary Microbiology 54: 1217–1226. DOI 10.1099/ijs.0.02823-0.
Sullivan, M. 2013. CPHST Pest Datasheet for ‘Candidatus Phytoplasma mali’. USDA-APHIS-PPQ-CPHST.
Seemüller, E., and Schneider, B. 2007. Differences in virulence and genomic features of strains of ‘Candidatus Phytoplasma mali’, the apple proliferation agent. Phytopathology 97:964-970. doi:10.1094/PHYTO-97-8-0964.
Abraitienë, A., Bevilacqua, A., Scarafoni, A., and Quaglino, F. 2018. First report of Forsythia suspensa, Spiraea vanhouttei and Viburnum lantana as new natural plant hosts of ‘Candidatus Phytoplasma mali’, the causal agent of apple proliferation disease, in Lithuania. Plant Disease (accepted for publication). doi:10.1094/PDIS-01-18-0090-PDN.
Sullivan, M. 2013. CPHST Pest Datasheet for ‘Candidatus Phytoplasma prunorum’. USDA-APHIS-PPQ-CPHST. Revised July 2014.